
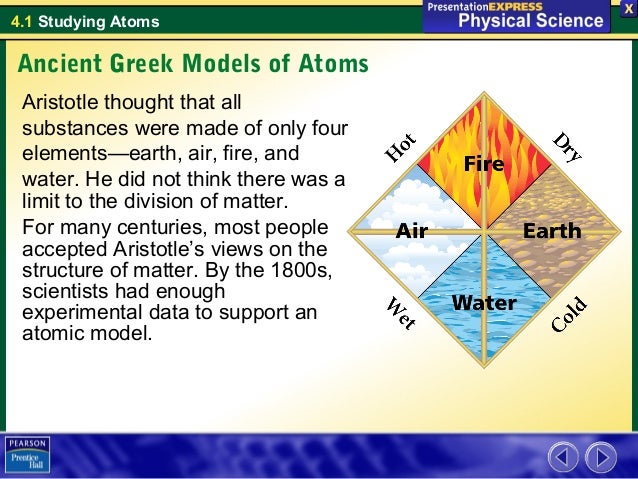
Some of these figures are treated in more depth in other articles in this encyclopedia: the reader is encouraged to consult individual entries on Leucippus, Democritus. The Bohr atomic model later replaced the Rutherford model. A number of important theorists in ancient Greek natural philosophy held that the universe is composed of physical ‘atoms’, literally ‘uncuttables’. He taught his beliefs, and he was so well known for his teachings, most believed him to be right. He was a Greek philosopher who introduced core ideas to science. He believed that all substances were made of Fire, Water, Air, and Earth. The first atomic theories were introduced around the 6th century BC by Leucippus. Rutherford linked this motion to the orbit of planets around the sun. There was no model that he revised or created because when the Atomic Theory was discovered, Aristotle did not believe in it. The angle of deflection from the particles also showed that there was most likely a strong positively charged nucleus in the middle of the atom with negatively charged particles circling around it. This made the most sense, since it explained why so few particles were hitting the gold foil. Aristotle, for instance, disagreed completely, stating instead that matter was made of four elements: earth, wind, water and fire, and later scientists followed. As a result, Rutherford created a theory that stated that most of an atom was empty space. Only about one in 8,000 was deflected away into the surrounding detecting screen. Through this experiment, Rutherford determined that the vast majority of the particles he fired at the gold foil passed right through it. The detecting screen had zinc sulfide in it to allow Rutherford to detect the presence of particles after they passed through the filtering gold foil. Aristotle believed that universal forms did not have to be attached to each object or concept, and that every instance of an object or concept had to be examined on its own.Ernest Rutherford’s gold foil experiment involved a particle emitter, a round detecting screen with a slit in it and a slip of gold foil in the middle. In his remarkable life Empedocles devised a theory of natural selection proposed that everything in existence is made of different combinations of four elements: air, fire, wind and earth recognized that air has weight said that the speed of light is finite and made a statement equivalent to the. Plato believed that concepts had a universal and ideal form, which led to his idealistic philosophy. Atomic Theory As was written, all matter except dark matter is made of molecules, which are themselves made of atoms. Empedocles lived 2500 years ago, soon after the dawn of scientific thought in Ancient Greece. What is the difference between Plato and Aristotle? However, prior to the scientific revolution and the development of the scientific method starting in the 16th century, ideas about. Instead, he claimed that forms are intrinsic to objects and cannot exist apart from them, so they must be studied in relation to them. A theory of the structure and behavior of atoms has taken more than two millenia to evolve, from the abstract musings of ancient Greek philosophers to the high-tech experiments of modern scientists. Plato’s theory of forms, which claims that properties like beauty are abstract universal entities that exist independently of the objects themselves, was famously rejected by Aristotle. Compounds are formed when atoms are combined in simple ratios to form compound atoms (molecules). According to the theory, matter is made up of indivisible particles known as atoms, and that all atoms of a given element are identical and cannot be created or destroyed.


 0 kommentar(er)
0 kommentar(er)
